Shared Goals
The AMR Industry Alliance’s Commitments
The AMR Industry Alliance ensures that signatories from biotech, diagnostics, generics and research-based pharmaceutical companies collectively deliver on the specific commitments made in the Declaration and the Roadmap and will measure progress made in the fight against AMR.

RESEARCH & SCIENCE
- Invest in research and development for innovative antibiotics and antibiotic dosage forms, vaccines, new technologies, and diagnostics.
- Continue to advocate for policies that support sustainable investment in AMR-relevant innovation.
- Partner with policymakers, payers, and other relevant stakeholders on new reimbursement, valuation, and commercial models that support appropriate patient access and a sustainable supply of antibiotics and AMR-relevant vaccines and new technologies and diagnostics.
- Support collaboration and sharing of relevant non-proprietary data with different stakeholders (e.g., academia, consortia, small or medium-sized enterprise (SMEs), public researchers and industry) to help address key scientific and public health challenges.

ACCESS
- Address barriers to patient access to the most appropriate treatment, vaccine, or diagnostic.
- Work in collaboration with policymakers to create an economic and regulatory environment that enables the sustainable supply of quality-assured antibiotics.
- Work to reduce the prevalence of substandard and falsified AMR-relevant products.

APPROPRIATE USE
- Contribute to slowing the emergence of resistance by preventing infections by promoting vaccination and reduction of inappropriate use of antibiotics through expanded use of diagnostics.
- Support appropriate use of antibiotics by working closely with other partners on awareness campaigns, continued education for healthcare professionals, and generation of evidence to support appropriate use and stewardships.
- Collect and share surveillance data with public health bodies and healthcare professionals to improve understanding of resistance trends, monitor the effectiveness of antibiotics, inform appropriate antibiotic and vaccine use, and develop adapted infection control strategies.
- Ensure that any promotional activities for antibiotics are aligned with the goal of advancing stewardship.

MANUFACTURING AND ENVIRONMENT
- Review Alliance member’s own manufacturing and supply chains to assess good practice in controlling release of antibiotics into the environment.
- Establish a common framework for managing antibiotic discharge, and start to apply it across their own manufacturing and supply chains by 2018.
- Work with stakeholders to develop a practical mechanism transparently show that Alliance member supply chains meet the frameworks standards.
- Work with independent technical experts to establish science-driven, risk-based targets for discharge concentrations of antibiotics and develop good practice methods to reduce environmental impacts of manufacturing discharges by 2020.
We measure and drive the life sciences industry progress to curb antimicrobial resistance in four different areas:

research & science
Invest in R&D to meet public health needs with new innovative diagnostics and treatments

appropriate use
Work to reduce the development of antimicrobial resistance

access
Improve access to high-quality antibiotics and ensuring that new ones are available to all

manufacturing
Reduce the environmental impact of manufacturing
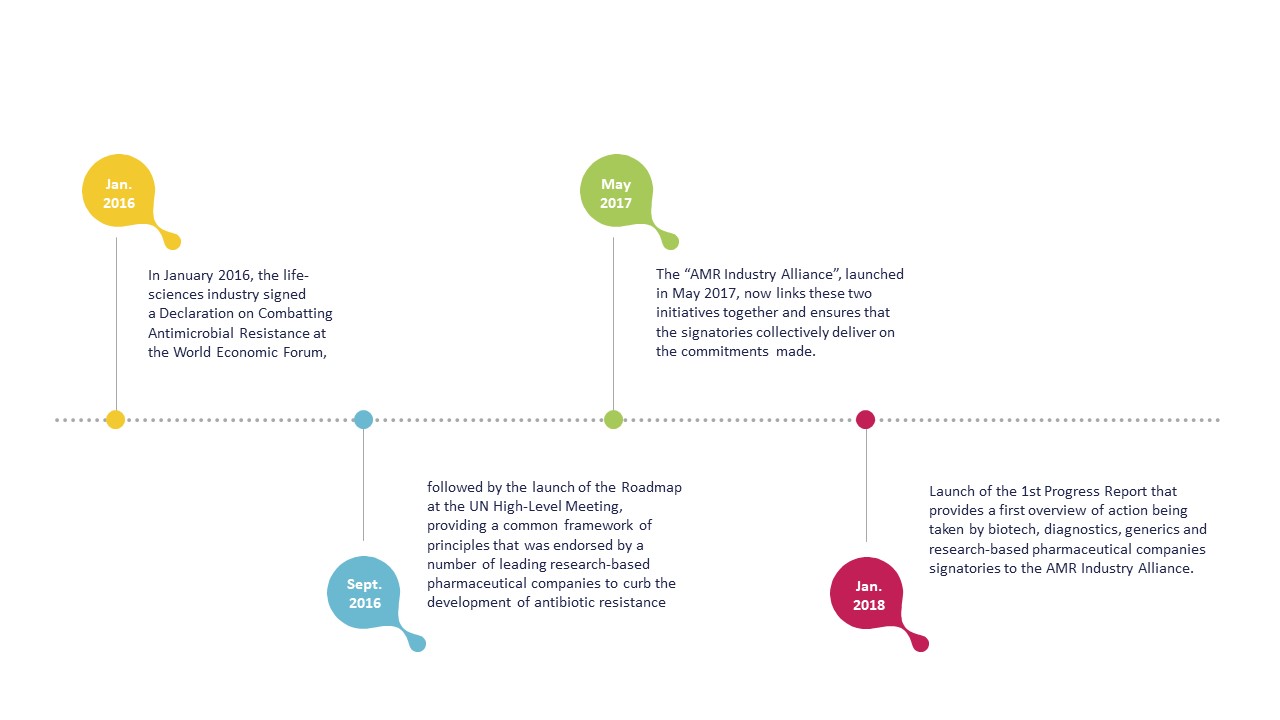
Declaration – January 2016
The Declaration sets out a common set of principles for global action that focus on reducing the development of AMR, investing in R&D to meet public health needs and improving access to antibiotics, vaccines and diagnostics. The Declaration also aims at encouraging governments to go beyond political statements. The Declaration specifically calls on governments to commit to allocating the funds needed to create a sustainable market for antibacterials while also implementing measures to prevent infection and improve appropriate use.
Signatories: Over 100 biotech, diagnostics, generics and research-based pharmaceutical companies and associations have signed the Declaration.
Commitments:
- Work to reduce the development of AMR
- Invest in R&D to meet public health needs with new innovative diagnostics & treatments
- Improve access to existing high-quality antibiotics and ensuring that new ones are available to all
Roadmap – September 2016
The Roadmap was launched by a group of leading companies in order to drive progress on four key commitments as applicable according to their different businesses and capabilities. These are in line with the Declaration, and reflect the companies’ intent to continue to proactively contribute to the global efforts to address AMR. The signatories of the Roadmap are striving to be at the forefront of the fight against AMR and lead by example.
Signatories: A sub-set of companies committed to the Industry Declaration: 13 companies of the 100+ Alliance members are committed to the Roadmap.
Commitments: The Roadmap lays out four commitments to reduce AMR by 2020: reduce the environmental impact of manufacturing, addressing inappropriate use, improving global access and developing a broad R&D ecosystem.
